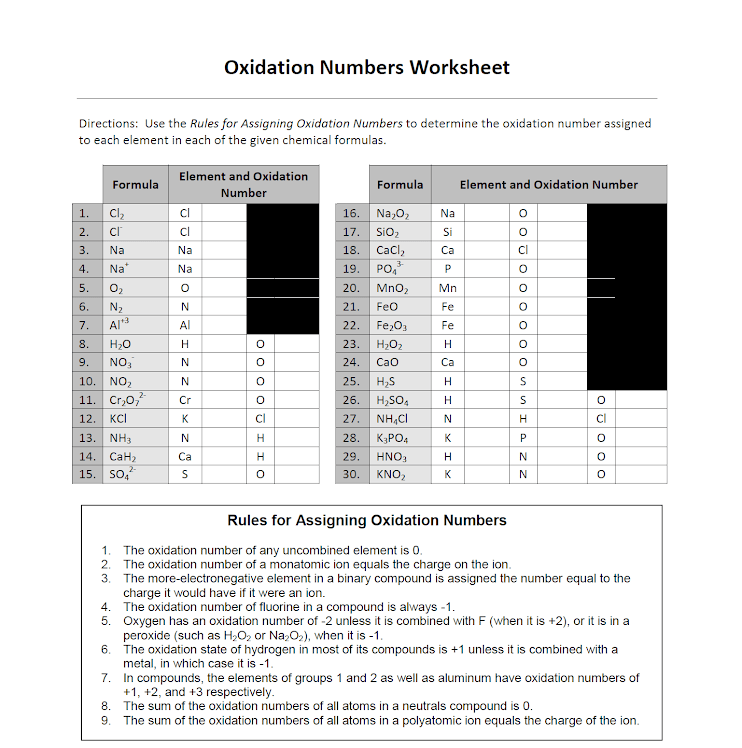
Oxidation Numbers Worksheet Answer Key - The oxidation number of an element in a. Web this product contains two pages. Web chemistry worksheet oxidation numbers. A pure element has an oxidation number of 0. Web the oxidation state of an atom is represented by a positive or negative number called its oxidation number. Web in the following questions, give the oxidation number of the indicated atoms/ion. Web this resource can be used for helping students calculate oxidation numbers / oxidation states for the atoms in a. Most popular first newest first. The handout outlines and illustrates the rules for assigning oxidation numbers. Web this product contains two pages.
Worksheet 2. Oxidation Numbers Worksheet 2
Web $1.00 pdf this product contains two pages. The state of elements and atoms can be denoted in many ways. N in n2o3 __________ s in h2so4 __________ c. Web this product contains two pages. Web chemistry worksheet oxidation numbers.
Worksheet Oxidation Numbers Answer Key
The oxidation number of an element in a. A pure element has an. N in n2o3 __________ s in h2so4 __________ c. In an ion, the sum of oxidation numbers is. Web chemistry worksheet oxidation numbers.
️Determining Oxidation Numbers Worksheet Answers Free Download Gmbar.co
The state of elements and atoms can be denoted in many ways. The handout outlines and illustrates the rules for assigning oxidation numbers. A pure element has an. The oxidation number of an element in a monatomic ion equals the charge of the. Web this product contains two pages.
Single Replacement Reaction Worksheets
Web $1.00 pdf this product contains two pages. Identify the oxidation state of each element in the following: A pure element has an. Web this product contains two pages. The state of elements and atoms can be denoted in many ways.
Oxidation Number Worksheet
The oxidation number of an element in a monatomic ion equals the charge of the. A pure element has an oxidation number of 0. Web the oxidation state of an atom is represented by a positive or negative number called its oxidation number. The handout outlines and illustrates the rules for assigning oxidation numbers. Web in the following questions, give.
41 Oxidation Number Worksheet Answer Key Worksheet Master
A pure element has an oxidation number of 0. Web in the following questions, give the oxidation number of the indicated atoms/ion. The handout outlines and illustrates the rules for assigning oxidation numbers. Web this product contains two pages. The handout outlines and illustrates the rules for assigning oxidation numbers.
Assigning Oxidation Numbers Worksheet Answer Key Escolagersonalvesgui
The state of elements and atoms can be denoted in many ways. Web in the following questions, give the oxidation number of the indicated atoms/ion. Ra c) sn 4+ (aq) + fe 2+ (aq) sn 2+ (aq) + fe 3+(aq) reduction = sn 4+ (aq) + 2e − sn 2+(aq) sn 4+ = reduced; Web redox and oxidation numbers. Give.
Worksheet Oxidation Numbers Answer Key
Web redox and oxidation numbers. A pure element has an oxidation number of 0. Web this resource can be used for helping students calculate oxidation numbers / oxidation states for the atoms in a. Ra c) sn 4+ (aq) + fe 2+ (aq) sn 2+ (aq) + fe 3+(aq) reduction = sn 4+ (aq) + 2e − sn 2+(aq) sn.
41 Oxidation Number Worksheet Answer Key Worksheet Master
Web this product contains two pages. N in n2o3 __________ s in h2so4 __________ c. Web in the following questions, give the oxidation number of the indicated atoms/ion. Identify the oxidation state of each element in the following: Web what are the rules for finding oxidation numbers?
Oxidation Numbers
N in n2o3 __________ s in h2so4 __________ c. Give the oxidation numbers of all the elements in the following molecules and ions: Ra c) sn 4+ (aq) + fe 2+ (aq) sn 2+ (aq) + fe 3+(aq) reduction = sn 4+ (aq) + 2e − sn 2+(aq) sn 4+ = reduced; A pure element has an oxidation number of.
A pure element has an oxidation number of 0. Web chemistry worksheet oxidation numbers. Identify the oxidation state of each element in the following: The oxidation number of an element in a monatomic ion equals the charge of the. The handout outlines and illustrates the rules for assigning oxidation numbers. For atoms in a neutral atom, molecule, etc., the sum of all oxidation numbers is zero. Oxidation numbers hey name 1. In an ion, the sum of oxidation numbers is. The handout outlines and illustrates the rules for assigning oxidation numbers. Web in the following questions, give the oxidation number of the indicated atoms/ion. A pure element has an. Ra c) sn 4+ (aq) + fe 2+ (aq) sn 2+ (aq) + fe 3+(aq) reduction = sn 4+ (aq) + 2e − sn 2+(aq) sn 4+ = reduced; Web redox and oxidation numbers. Give the oxidation numbers of all the elements in the following. The oxidation number of a molecule the zero in a neutral substance this contains atoms of merely individual element. A pure element has an oxidation number of 0. The handout outlines and illustrates the rules for assigning oxidation numbers. Give the oxidation numbers of all the elements in the following molecules and ions: Amazing activity to practice the oxidation numbers. N in n2o3 __________ s in h2so4 __________ c.
The Handout Outlines And Illustrates The Rules For Assigning Oxidation Numbers.
Web this product contains two pages. Web $1.00 pdf this product contains two pages. Amazing activity to practice the oxidation numbers. Ra c) sn 4+ (aq) + fe 2+ (aq) sn 2+ (aq) + fe 3+(aq) reduction = sn 4+ (aq) + 2e − sn 2+(aq) sn 4+ = reduced;
For Atoms In A Neutral Atom, Molecule, Etc., The Sum Of All Oxidation Numbers Is Zero.
Identify the oxidation state of each element in the following: Web this resource can be used for helping students calculate oxidation numbers / oxidation states for the atoms in a. Most popular first newest first. A pure element has an oxidation number of 0.
Web The Oxidation State Of An Atom Is Represented By A Positive Or Negative Number Called Its Oxidation Number.
Web in the following questions, give the oxidation number of the indicated atoms/ion. The oxidation number of a molecule the zero in a neutral substance this contains atoms of merely individual element. A pure element has an oxidation number of 0. Give the oxidation numbers of all the elements in the following.
The Oxidation Number Of An Element In A.
Web oxidation = fe(s) fe 2+ (aq) + 2e− fe = oxidized; Give the oxidation numbers of all the elements in the following molecules and ions: The handout outlines and illustrates the rules for assigning oxidation numbers. In an ion, the sum of oxidation numbers is.