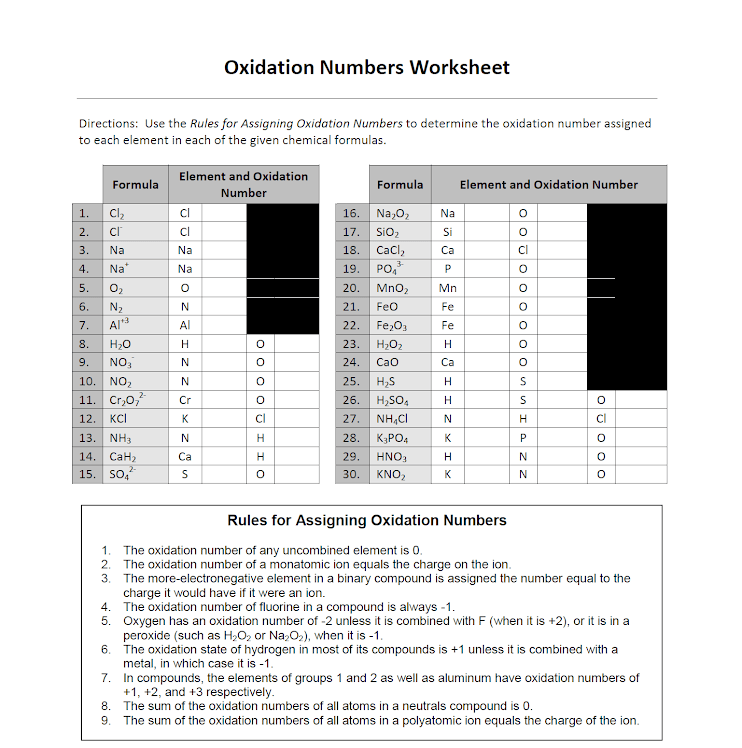
Oxidation Numbers Worksheet Answers - Amazing activity to practice the oxidation numbers. Web complete the ‘oxidation number’ column of the table below by working out the oxidation number of each of the transition metal cations. Identify the oxidation state of nitrogen in the following: In this oxidation numbers worksheet, students. Use the rules for assigning oxidation numbers to determine the oxidation number assigned to each. The oxidation number of an element in its free (uncombined) state is zero — for example, al(s) or zn(s). Web the oxidation state of an atom is represented by a positive or negative number called its oxidation number. N in n2o3 __________ s in h2so4. Web assign oxidation numbers to the atoms in each substance. Such is impossible for vanadium, but can.
Assigning Oxidation Numbers Worksheet Answers
Web rules for assigning oxidation numbers. Web the oxidation state of an atom is represented by a positive or negative number called its oxidation number. Web oxidation number worksheet with answers. Cdrom index 30sw discussion of answers: This has been completed for the complexes in the first two rows of the table.
oxidation numbers worksheet
Web the oxidation state of an atom is represented by a positive or negative number called its oxidation number. Web oxidation numbers worksheet directions: The oxidation number is the charge that a central metal atom will. Web rules for assigning oxidation numbers. Use the rules for assigning oxidation numbers to determine the oxidation number assigned to each.
️Determining Oxidation Numbers Worksheet Answers Free Download Gambr.co
Cdrom index 30sw discussion of answers: In this oxidation numbers worksheet, students. In the following questions, give the oxidation number of the indicated atoms/ion. Most popular first newest first. Use the rules for assigning oxidation numbers to determine the oxidation number assigned to each.
Charting Oxidation Number Worksheet Answer Key Worksheets Joy
Web oxidation number worksheet with answers. Cdrom index 30da topics working out oxidation numbers. Web the oxidation state of an atom is represented by a positive or negative number called its oxidation number. In the following questions, give the oxidation number of the indicated atoms/ion. Such is impossible for vanadium, but can.
️Determining Oxidation Numbers Worksheet Answers Free Download Gambr.co
Web oxidation number worksheet with answers. Web rules for assigning oxidation numbers. Web assign oxidation numbersto each of the atoms in the following compounds: Web assign oxidation numbers to the atoms in each substance. N in n2o3 __________ s in h2so4.
️Determining Oxidation Numbers Worksheet Answers Free Download Gmbar.co
In this oxidation numbers worksheet, students. Identify the oxidation state of nitrogen in the following: Cdrom index 30sw discussion of answers: Complete the ‘d configuration’ column in the table by working out z for each of the transition metal ions. Amazing activity to practice the oxidation numbers.
Assigning Oxidation Numbers Worksheet Answer Key Escolagersonalvesgui
Give the oxidation numbers of all the elements in the following. Web assign oxidation numbersto each of the atoms in the following compounds: Pcl 5 (nh 4) 2 se ag li 2 o 2 assign oxidation numbers to the atoms in each substance. Web the oxidation number chemistry worksheet will help you to learn about the oxidation number of atoms..
Worksheet 2. Oxidation Numbers Worksheet 2
In this oxidation numbers worksheet, students. P 4 so 3 so 3 2 − ca 3 (po 3) 2 assign oxidation numbers to the atoms in each substance. Amazing activity to practice the oxidation numbers. Complete the ‘d configuration’ column in the table by working out z for each of the transition metal ions. Cdrom index 30da topics working out.
Oxidation Number Worksheet
Web oxidation number worksheet with answers. Cdrom index 30sw discussion of answers: P 4 so 3 so 3 2 − ca 3 (po 3) 2 assign oxidation numbers to the atoms in each substance. Web if electrons are added in an elemental species, its oxidation number turn negative. Web the oxidation number chemistry worksheet will help you to learn about.
17 Organic Oxidation Reactions Worksheet /
Web assign oxidation numbers to the atoms in each substance. In the following questions, give the oxidation number of the indicated atoms/ion. Most popular first newest first. The oxidation number of any uncombined element is 0. N in n2o3 __________ s in h2so4.
Oxidation numbers hey name 1. Web assign oxidation numbers to the atoms in each substance. Web the oxidation state of an atom is represented by a positive or negative number called its oxidation number. Give the oxidation numbers of all the elements in the following. Web rules for assigning oxidation numbers. Web assign oxidation numbersto each of the atoms in the following compounds: This has been completed for the complexes in the first two rows of the table. The oxidation number is the charge that a central metal atom will. Web if electrons are added in an elemental species, its oxidation number turn negative. Web introduction the concept of oxidation numbers, or oxidation states (on), was designed to determine whether or not. The “unknown” oxi dation state is the number that must be added to the total of the “known” oxidation states to make the total of the oxidation. The oxidation number of an element in its free (uncombined) state is zero — for example, al(s) or zn(s). Use the rules for assigning oxidation numbers to determine the oxidation number assigned to each. Web oxidation numbers worksheet directions: Cdrom index 30sw discussion of answers: Web the oxidation number chemistry worksheet will help you to learn about the oxidation number of atoms. Web complete the ‘oxidation number’ column of the table below by working out the oxidation number of each of the transition metal cations. In the following questions, give the oxidation number of the indicated atoms/ion. Most popular first newest first. Web oxidation number worksheet with answers.
Web Rules For Assigning Oxidation Numbers.
In this oxidation numbers worksheet, students. P 4 so 3 so 3 2 − ca 3 (po 3) 2 assign oxidation numbers to the atoms in each substance. Identify the oxidation state of nitrogen in the following: Web if electrons are added in an elemental species, its oxidation number turn negative.
N In N2O3 __________ S In H2So4.
Web the oxidation state of an atom is represented by a positive or negative number called its oxidation number. The oxidation number is the charge that a central metal atom will. Such is impossible for vanadium, but can. In the following questions, give the oxidation number of the indicated atoms/ion.
Cdrom Index 30Da Topics Working Out Oxidation Numbers.
Amazing activity to practice the oxidation numbers. Web complete the ‘oxidation number’ column of the table below by working out the oxidation number of each of the transition metal cations. Oxidation numbers hey name 1. Web introduction the concept of oxidation numbers, or oxidation states (on), was designed to determine whether or not.
Use The Rules For Assigning Oxidation Numbers To Determine The Oxidation Number Assigned To Each.
The oxidation number of an element in its free (uncombined) state is zero — for example, al(s) or zn(s). This has been completed for the complexes in the first two rows of the table. Give the oxidation numbers of all the elements in the following. The “unknown” oxi dation state is the number that must be added to the total of the “known” oxidation states to make the total of the oxidation.