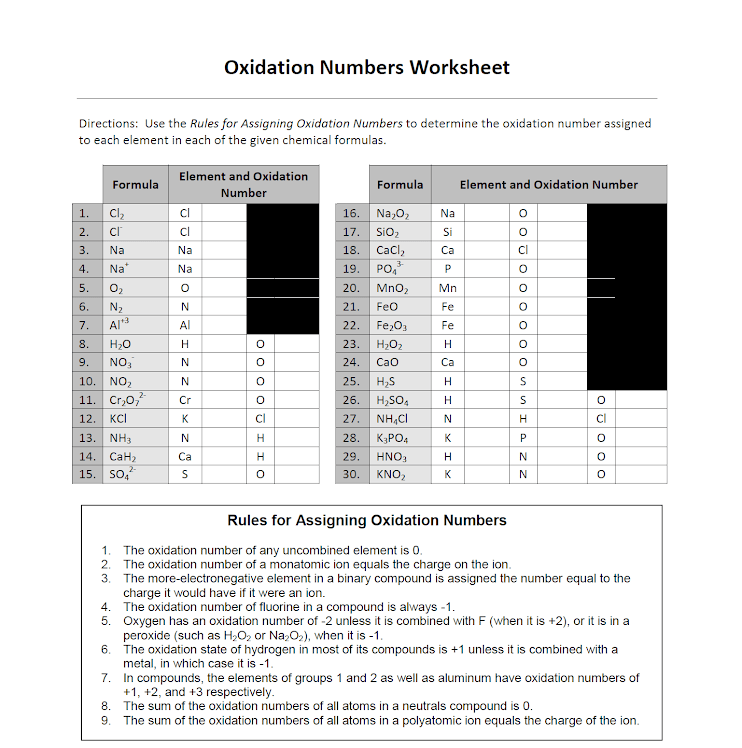
Worksheet Oxidation Numbers - Describe how this is achieved with 5 ligands. The charge on all metals of group 2 of the periodic. Web 2fe(s) + 3cl2(g) → 2fe3 + + 6cl −. Identify what is being oxidized and reduced in this redox reaction by assigning oxidation numbers to the atoms. Web rules for assigning oxidation numbers. Zn(c 2 h 3 o 2) 2; 2no + cl 2 → 2nocl N in n2o3 __________ s in h2so4 __________ c. Amazing activity to practice the oxidation numbers. Web introduction the concept of oxidation numbers, or oxidation states (on), was designed to determine whether or not.
Worksheet Oxidation Numbers Answer Key
The fe (iii) is released in a cell by a decrease in the ph. Web the oxidation state of an atom is represented by a positive or negative number called its oxidation number. Amazing activity to practice the oxidation numbers. The charge on all metals of group 2 of the periodic. Web mar 13, 2023 5b:
Assigning Oxidation Numbers Worksheet Fill Out and Sign Printable PDF
Web the oxidation state of an atom is represented by a positive or negative number called its oxidation number. Zn(c 2 h 3 o 2) 2; Web mar 13, 2023 5b: The charge on all free elements is zero. 2no + cl 2 → 2nocl
07 Finding Oxidation Numbers Worksheet
Amazing activity to practice the oxidation numbers. The charge on all metals of group 1 of the periodic table is +1 c. Describe how this is achieved with 5 ligands. Web 2fe(s) + 3cl2(g) → 2fe3 + + 6cl −. Web mar 13, 2023 5b:
41 Oxidation Number Worksheet Answer Key Worksheet Master
In essence, the fe pushes electrons and the cl 2 pulls electrons, thereby effecting. Assign oxidation numbersto each of the atoms in the following compounds: N in n2o3 __________ s in h2so4 __________ c. Identify what is being oxidized and reduced in this redox reaction by assigning oxidation numbers to the atoms. The worksheet will also help.
Worksheet Oxidation Numbers
The fe (iii) is released in a cell by a decrease in the ph. Describe how this is achieved with 5 ligands. 2no + cl 2 → 2nocl Web 2fe(s) + 3cl2(g) → 2fe3 + + 6cl −. In essence, the fe pushes electrons and the cl 2 pulls electrons, thereby effecting.
Oxidation Numbers Worksheet
Assign oxidation numbers to the atoms in each substance. Web oxidation numberfinding the oxidation number. Web in the following questions, give the oxidation number of the indicated atoms/ion. Web the oxidation state of an atom is represented by a positive or negative number called its oxidation number. Web oxidation numbers worksheet by jemunoz.
️Oxidation Numbers Worksheet Free Download Goodimg.co
The worksheet will also help. Web assign oxidation numbers to the atoms in each substance. N in n2o3 __________ s in h2so4 __________ c. Assign oxidation numbersto each of the atoms in the following compounds: The oxidation number of any uncombined element is 0.
Oxidation Numbers Worksheet Answers flakeinspire
Assign oxidation numbers to the atoms in each substance. Web in the following questions, give the oxidation number of the indicated atoms/ion. Web the oxidation number chemistry worksheet will help you to learn about the oxidation number of atoms. Web the oxidation state of an atom is represented by a positive or negative number called its oxidation number. What is.
Solved Oxidation Numbers Worksheet Directions Use the Rules
In essence, the fe pushes electrons and the cl 2 pulls electrons, thereby effecting. Web 2fe(s) + 3cl2(g) → 2fe3 + + 6cl −. Amazing activity to practice the oxidation numbers. Web oxidation numbers worksheet by jemunoz. Web in the following questions, give the oxidation number of the indicated atoms/ion.
Worksheet Assigning Oxidation Numbers Key.doc
What is the coordination number and approximate geometry of the fe (iii) atom. Amazing activity to practice the oxidation numbers. Zn(c 2 h 3 o 2) 2; The charge on all metals of group 1 of the periodic table is +1 c. Web oxidation numberfinding the oxidation number.
Web oxidation numbers worksheet by jemunoz. Web mar 13, 2023 5b: Web the oxidation state of an atom is represented by a positive or negative number called its oxidation number. The charge on all metals of group 1 of the periodic table is +1 c. Web introduction the concept of oxidation numbers, or oxidation states (on), was designed to determine whether or not. The charge on all metals of group 2 of the periodic. Web assign oxidation numbers to the atoms in each substance. The charge on all free elements is zero. Web 2fe(s) + 3cl2(g) → 2fe3 + + 6cl −. Web oxidation numberfinding the oxidation number. Identify what is being oxidized and reduced in this redox reaction by assigning oxidation numbers to the atoms. Zn(c 2 h 3 o 2) 2; 2no + cl 2 → 2nocl Iron complex in transferrin questions how many unpaired electrons will the fe (iii) atom have? Web rules for assigning oxidation numbers. Assign oxidation numbers to the atoms in each substance. In this oxidation numbers worksheet, students. Amazing activity to practice the oxidation numbers. In essence, the fe pushes electrons and the cl 2 pulls electrons, thereby effecting. The fe (iii) is released in a cell by a decrease in the ph.
Web Assign Oxidation Numbers To The Atoms In Each Substance.
Web the oxidation state of an atom is represented by a positive or negative number called its oxidation number. Web in the following questions, give the oxidation number of the indicated atoms/ion. Web oxidation numberfinding the oxidation number. 2no + cl 2 → 2nocl
Web Oxidation Numbers Worksheet By Jemunoz.
Iron complex in transferrin questions how many unpaired electrons will the fe (iii) atom have? What is the coordination number and approximate geometry of the fe (iii) atom. Web mar 13, 2023 5b: The charge on all metals of group 2 of the periodic.
The Oxidation Number Of Any Uncombined Element Is 0.
The worksheet will also help. Identify what is being oxidized and reduced in this redox reaction by assigning oxidation numbers to the atoms. Assign oxidation numbers to the atoms in each substance. In essence, the fe pushes electrons and the cl 2 pulls electrons, thereby effecting.
Assign Oxidation Numbersto Each Of The Atoms In The Following Compounds:
N in n2o3 __________ s in h2so4 __________ c. Web 2fe(s) + 3cl2(g) → 2fe3 + + 6cl −. Web introduction the concept of oxidation numbers, or oxidation states (on), was designed to determine whether or not. In this oxidation numbers worksheet, students.